Wolfgang Kuchinke, Coordination Centre for Clinical Trials (KKS) of the Heinrich-Heine University in Duesseldorf, Germany, and main interface for the ECRIN – EUDAT data pilot, explains how ECRIN advances the knowledge flow of research, the competiveness and the degree of integration of European clinical research. |
 |
The European Clinical Research Infrastructure (ECRIN) is a non-profit intergovernmental organisation that supports the conduct of multinational clinical trials in Europe by providing trial services. Clinical trials explore whether a medical strategy, treatment, or medical device is safe and effective for humans. Based in Paris, ECRIN is a network that uses European Correspondents to integrate the work of national networks of clinical trial units (CTUs) across Europe, including numerous international stakeholders involved in clinical research.
Obstacles for clinical research consist of missing infrastructure interoperability, regulatory and ethical requirements and management and funding issues, all of which deter especially academic investigators from beginning multinational trials. It was recognized therefore that support is needed to enable Europe to take advantage of its population size to access patients, and to unlock latent scientific potential and clinical expertise. As reaction to the need for a pan-European infrastructure to support clinical trials the European Union provided resources to create ECRIN in 2006. ECRIN has now the status of a European Research Infrastructure Consortium (ERIC), with member countries providing the financial support for maintaining the infrastructure.
About Dr Wolfgang Kuchinke:Dr Wolfgang Kuchinke is a trained biologist with expertise in clinical pharmacology. At the Coordination Centre for Clinical Trials (KKS) of the Heinrich-Heine University in Duesseldorf he managed clinical trials and has focused since on information technology using the internet for clinical research. This includes the evaluation of Electronic Data Capture (EDC) systems, employing CDISC standards for interoperability, and system validation for GCP compliance. He was involved in developing the clinical research infrastructure for ECRIN. His recent areas of research involve the collaboration between research infrastructures, the legal and semantic conditions for data sharing and the inclusion of sensitive data in research processes.
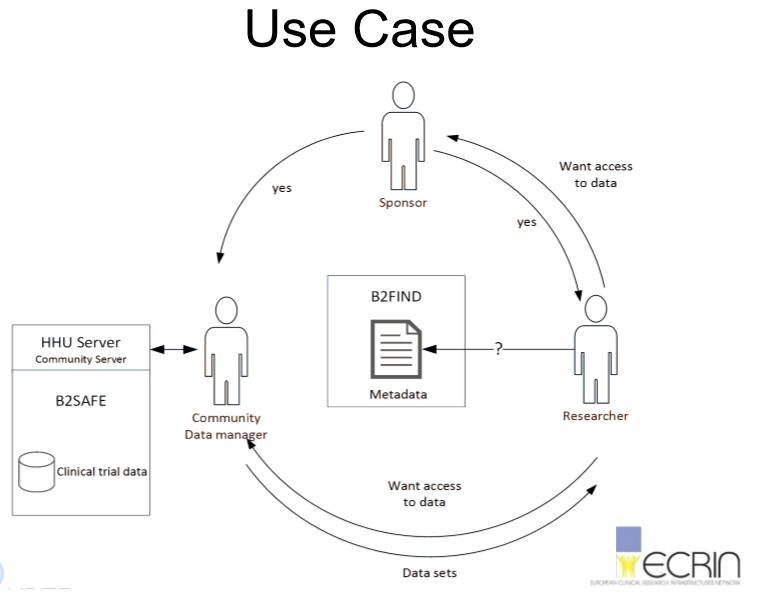
About ECRIN:ECRIN (the European Clinical Research Infrastructure) is a public, non-profit organisation that links scientific partners and networks across Europe to facilitate multinational clinical research (www.ecrin.org). Click here to find more information on the EUDAT – ECRIN pilot or download the presentation "EUDAT Pilot of the ECRIN Community: long-term storage of clinical trials data" to understand more about the state of the pilot.
About the EUDAT Working Group on Sensitive Data:The purpose of the “EUDAT Working Group on Sensitive Data” is to investigate the different requirements of communities related to sensitive data. Click here to find more information about the working group.